If answered yes to questions 1 and 2, take a moment to rule out these other diseases before confirming your CDM diagnosis. Keep in mind that these diseases are not mutually exclusive. More than one disease can affect a plant.
If you still suspect CDM, please make a report here or contact your local extension office or Plant Disease Clinic
Alternaria leaf blight (Alternaria cucumerina)
Alternaria produces small, circular yellow-brown leaf lesions. As lesions enlarge, they often develop a concentric ring pattern, which helps differentiate this disease from CDM. Stem lesions do not occur with this disease and fruit infection is rare.
Gummy stem blight (Didynella bryoniae)
Gummy stem blight leaf lesions are dark brown and often develop near margins. Gummy stem blight can be differentiated from CDM because it produces ovate stem and vine cankers near the nodes, with a characteristic brown gummy exudate.
Target Spot (Corynespora cassiicola)
Target spot begins on leaves as water-soaked angular lesions, which later become light brown and circular with a defined, dark brown margin. Older lesions can drop out, creating a shot-hole appearance, which does not occur with CDM. Signs of the fungus’ dark sporulation are difficult to see without magnification. Fruit can become infected.
Anthracnose (Colletotrichum orbiculare)
Anthracnose leaf lesions begin circular and become large and irregular. Unlike CDM, leaf lesions often occur near leaf veins. As anthracnose lesions dry, they often crack, creating a shot-hole appearance. Anthracnose produces stem lesions and fruit rot, whereas CDM does not. The anthracnose fungus may produce signs of black fruiting bodies and salmon pink spore masses.
Scab (Cladosporium cucumerinum)
Scab leaf lesions are initially water-soaked and gradually turn gray. These spots often fall out, creating a shot-hole appearance, which is not commonly seen with CDM. Unlike CDM, this disease can occur on petioles, stems and fruit. An olive-green sporulation may appear on fruit.
Phytophthora foliar blight and fruit rot (Phytopthora capsici)
Phytophthora capsici causes damping-off, foliar blight and fruit rot on cucurbits. Phytophthora blight foliar symptoms begin water-soaked turning chlorotic to necrotic with olive-green borders. Leaf lesions can expand rapidly and are typically between 5 mm and 5 cm or more, making them larger than CDM lesions.
Cercospora leaf spot (Cercospora citrullina)
Cercospora leaf spot causes small, circular or angular brown spots. Lesion margin may appear dark and have yellow halos or large chlorotic areas surrounding them. Lesions often have thin, tan centers that drop out. Unlike CDM, Cercospora can affect the petioles and stems, and a fruit rot has been reported postharvest. Signs of the pathogen are difficult to see without magnification.
Septoria leaf spot (Septoria cucurbitacearum)
Septoria leaf lesions are small (1-2 mm in diameter) and can be water-soaked or brown under wet moist conditions or white under dry conditions. Signs of black fruiting bodies may be visible on older lesions with magnification. This disease can cause raised bumps on fruit rinds, which helps differentiate it from CDM.
Field Identification of Selected Cucurbit Diseases with a Hand Lens
This section describes techniques by which field personnel carrying a 10x to 20x hand lens (loupe) can make a tentative diagnosis based on symptoms (and fungal structures, if present).
The use of a compound microscope at 100x or 400x magnification to observe details of the pathogen is necessary for a positive identification.
Tips for Use of a Hand Lens
Hand lenses can be difficult to use because of the close working distance from the lens to the specimen and the small field of view. For a 10x lens, the working distance is approximately 25mm with a field view of 25mm in diameter; for a 20x lens, the field of view is less than half that diameter. At these magnifications, small movements of the specimen in relation to the lens and to the eye must be avoided. In order to take advantage of the full field of view, the lens must be held very close to the eye, centered over the pupil. A right-handed person should hold the lens between the index finger and thumb of the right hand, so that the lens is centered over the pupil of the left eye, about one eyelash away (6mm). The lens is steadied by resting the middle joints of the thumb and index finger on the bridge of the nose. Eyeglasses should not be worn. The specimen is comfortably held in the left hand and is moved close to the lens for focusing and scanning the field; the lens is not moved. For detail work, the specimen can be steadied in relationship to the lens by placing the little finger of the right hand on the a finger holding the specimen.
For lateral viewing of an infected leaf, the infected portion is folded over the left index finger and viewed from the side in such a way that the host tissue is in the lower portion of the field of view and the sky or other distant objects are in the upper portion of the field of view. Epidermal hairs show up nicely. Fungi with aerial spores, such as Alternaria spp., or with spores atop conidiophores, such as Corynespora cassiicola can be seen clearly. Typically, these structures are less than one-fourth of the length of leaf hairs. Fungi with fruiting bodies, such as Phoma and Colletotrichum, are best viewed from the top.
Fig. 1. Angular leaf spot caused by Pseudomonas syringae pv. lachrymans.
- Overhead view (10x) of a lesion, showing its angularity and the shiny, shellac-like appearance of the leaf surface due to dried bacterial ooze.
- Leaf section from a leaf spot mounted in a drop of water under a cover slip, with a bacterial ooze at the cut edge (40x).
Fig. 2. Alternatia leaf blight, caused by Alternaria cucumerina.
- Lateral view (20x) of a lesion, showing single, dark conidia on conidiophores. A leaf hair is shown for size comparison. Lesions occur on the upper and lower surfaces of leaves. The thick part of the spore adjoins the conidiophore, and the beak bears another spore.
- Conidia and a conidiophore (400x). Note the length of the beaks of conidia with longitudinal and transverse cross-walls.
Fig. 3. Anthracnose caused by Colleotrichum orbiculare.
- Lateral view (20x) of a lesion on the upper surface of a leaf, showing slightly raised, salmon-colored acervuli with one of three black, hair-like setae protruding from the surface. A leaf hair is shown for size comparison.
- Acervulus with three setae and hyaline conidia (400x).
Fig. 4. Downy mildew caused by Pseudoperonospora cubensis.
- Lateral view (20x) of the underside of a leaf lesion, showing a cluster of sporangiophores bearing purple conidia. A leaf hair is shown for size and comparison.
- Characteristic dichotomously branched, treelike sporangiophore bearing three sporangia, each with a papilla on the distal end (400x).
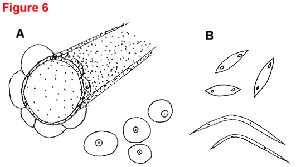
Fig. 5. Gummy stem blight caused by Didymella byroniae (anamorph Phoma cucurbitacearum).
- Overhead view (20x) of four round, slightly raised, tan pycnidia, each with a prominent ostiole (pore) in the center. A leaf hair is shown for size comparison.
- Two pycnidia releasing spore masses in twisting, snakelike chains (100x); three single-celled, hyaline conidia (400x).
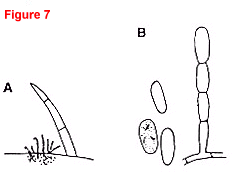
Fig. 6. Phomopsis black rot of cucumber caused by Phomopsis cucurbitae.
- Overhead view (20x) of four embedded and protruding pycnidia with prominent ostioles. A leaf hair is shown for size comparison. The pycnidia cannot be distinguished from those of Phoma cucurbitacearum, the gummy stem blight pathogen.
- Typical football-shaped alpha conidia, each with an oil droplet at each end, and long, thin, hairlike beta conidia with a prominent bend near the middle (400x).
Fig. 7. Powdery mildew caused by Sphaerotheca fuliginea.
- Laterial view (20x) of infected tissue, showing a cluster of long, white chains of conidia in a white colony. A leaf hair is shown for size comparison. Colonies form on succulent green tissue, leaves, and stems.
- Three mature conidia – one containing rod-shaped fibrosin bodies, and a simple, short conidiophore bearing three immature conidia in a chain (400x).
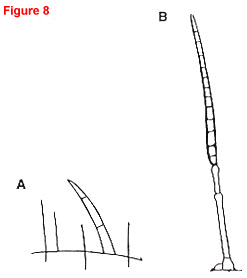
Fig. 8. Target leaf spot caused by Corynespora cassiicola.
- Lateral view (20x) of a lesion with four dark, conidia on long, straight, black conidiophores. A leaf hair is shown for size comparison. Conidiophores are produced on the upper and lower surfaces of leaves.